Gene Doping: Fiction or Future?
Introduction
For decades, scientists have been battling to find safe and effective gene therapy techniques for use in humans. Progress in the field has been slow but the prospect of the approach becoming an everyday therapeutic tool is edging closer. As with other medical advances, the development of gene therapy has not gone unnoticed by those seeking novel ways to enhance performance in sport – by way of gene doping.
This article examines what gene doping is, what demand there is for it, how it might work, and what might deter athletes from attempting it.
What is gene doping?
In very broad terms, “gene doping” can be considered as the use of techniques developed for gene therapy (which involves the introduction of foreign genetic material into a person's cells to prevent or fight disease) in order to improve athletic performance.
The idea of gene doping became particularly prominent following the publication in 1998 of a study co-authored by Professor Lee Sweeney involving the protein known as “IGF-1”, which was known to increase muscle growth. Investigating a treatment for age-related muscular deterioration, the study involved the insertion of additional copies of the IGF-1 gene into the hind legs of mice. Following the treatment, the mice showed an increase in strength of up to 27% and an increase in muscle mass of up to 19%. Perhaps unsurprisingly, the potential for using the technique to enhance athletic performance attracted significant attention.
Is there a demand for gene doping?
Whilst (at the time of writing) there are no reported cases of actual gene doping, there is evidence of the desire to use it. For example, in 2004, Thomas Springstein – a former German Athletics Association coach – was arrested on suspicion of providing performance enhancing drugs to minors. Following a search of his property, police officers found emails from him to the doctor of a Dutch speed-skating club, purportedly stating:
The new Repoxygen is hard to get. Please give me new instructions soon so that I can order the product before Christmas.
Repoxygen was a gene therapy product designed for use by anaemic patients, to increase red blood cell production to normal levels. Springstein’s hope, presumably, was that its use in healthy athletes would increase red blood cell numbers above this ‘normal’ level, resulting in an increased capacity to transport oxygen around the body to the muscles, and thereby enhancing work capacity. At the time of Springstein’s request, Repoxygen had not been tested on humans and its development has since been discontinued.

Further, there have been reports of athletes approaching scientists involved in gene therapy research. For example, Professor Sweeney – an author of the famous 1998 IGF-1 gene mice study – has said:
No matter what I say to [enquiring athletes] about [gene doping] being dangerous and experimental, it doesn't slow them down—they just keep pushing, saying, 'I want to be the guinea pig, I want to be the first person you try this on.' I kindly just say, 'look, it's not possible, I can't do it.'

How could gene doping work?
In order to understand how gene doping might work, it is useful to have a general understanding of what genes are and how they function.
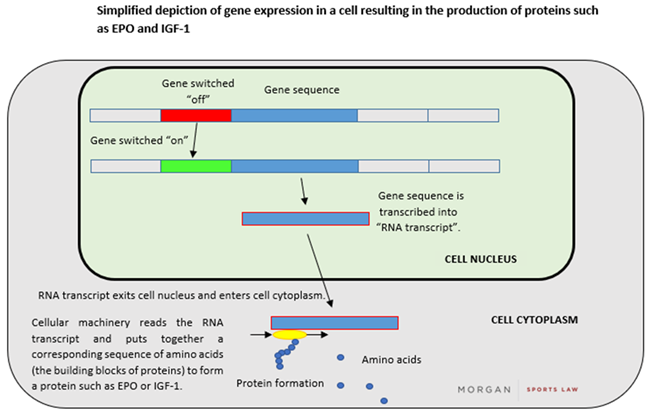
 A gene refers to a specific sequence of DNA letters that encode an individual functional unit or protein (such as erythropoietin (“EPO”), and, to use the example above, IGF-1). In human cells, a gene comprising double-stranded DNA is “transcribed” into a single-stranded "RNA transcript" which can be used as a template for the assembly of proteins – a process otherwise known as “gene expression”. A simplified depiction of this process is set out below:
A gene refers to a specific sequence of DNA letters that encode an individual functional unit or protein (such as erythropoietin (“EPO”), and, to use the example above, IGF-1). In human cells, a gene comprising double-stranded DNA is “transcribed” into a single-stranded "RNA transcript" which can be used as a template for the assembly of proteins – a process otherwise known as “gene expression”. A simplified depiction of this process is set out below:
By introducing new genetic material or interfering with existing genetic material, it is possible to manipulate genetic processes so that an athlete’s body produces a different amount of a certain protein, or even produces different proteins entirely. The simplified examples of two potential approaches to gene doping described below represent the tip of the iceberg in what is now a rapidly developing field. All of the methods described would be banned by the World Anti-Doping Agency (“WADA”).
Example method 1: increasing abundance of performance-enhancing gene products
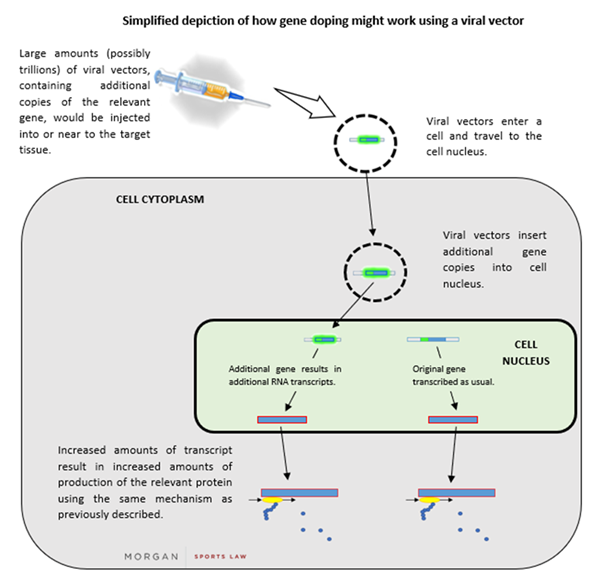
If a protein is advantageous for performance, it follows that more of that protein might allow for even better performance. There are numerous methods to increase the cellular abundance of a given protein, but one of the simplest methods involves delivering new genetic material into human cells. Such material can be introduced within a vehicle to carry it, such as a viral “vector” (a virus that has been partially disabled to minimise its harmful effects). Many viruses function by inserting their genetic material into the nuclei of host cells and co-opting the host’s protein production machinery to work on the newly inserted viral genes. This adaptation can be exploited to import and express the desired gene in the cells of athletes (in the same way as this adaption can be used in gene therapy).
After the relevant gene has been inserted into a viral vector, the vector could then be injected into a human (in large numbers). The vector then makes its way to a cell nucleus and inserts its DNA into it. The cell’s machinery then transcribes the additional gene which is within the newly-inserted vector DNA, resulting in increased quantities of the corresponding protein being produced (via the process described above). A simplified depiction of this process is set out below:
The method described above has been applied to monkeys in relation to the protein EPO – which is a hormone naturally produced by the kidneys, and which increases the production of red blood cells (in humans as well as monkeys). As red blood cells carry oxygen to the muscles, an increase in the number of red blood cells boosts the amount of oxygen delivered to the muscles and therefore the amount of work the muscles can do. There is a long history of EPO abuse in sport, although the final hormone product has traditionally been administered directly by injection. By transferring additional copies of genes coding for EPO, cells can be manipulated to overexpress the EPO gene and therefore produce more of the hormone. Indeed, in the study in question involving monkeys, this technique resulted in a dramatic increase in the volume of red blood cells as a proportion of the monkeys’ blood volume from around 40% to over 60% – with levels remaining above baseline for at least the duration of the six-month study.
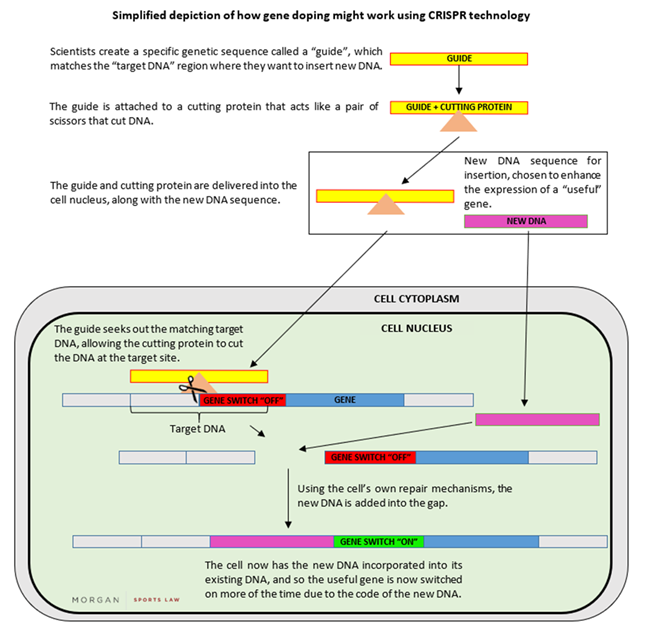
Instead of introducing entirely new copies of a gene in the manner described above, it is also possible to tweak the DNA around that gene to tune its expression level up. In other words, without inserting any new copies of a gene, the cell produces more of the RNA transcript. For example, in a study on mice, researchers edited a non-coding section of the mouse genome by inserting a sequence of DNA that increased the expression of the existing IGF-1 gene. The mice had a dramatic increase in their IGF-1 production and a number of the animals exhibited “clear muscle hypertrophy”. The technique used "CRISPR" which has been described as heralding a new dawn for gene therapy, given its scope for precisely editing a cell’s existing DNA. Indeed, a Chinese scientist recently claimed that he has created the first gene-edited babies, using CRISPR to disable a gene that allows the HIV virus to infect cells – which caused significant backlash globally. A simplified depiction of how gene doping might work using CRISPR technology is set out below:
Example method 2: decreasing abundance of performance-limiting gene products
In contrast to the genes referred to under example method 1 above, there are genes whose expression might impede athletic performance – so by removing or reducing the cellular abundance of the products of these genes, athletes might be able to break the limits on their performance. The gene encoding the protein myostatin – a negative regulator of muscle growth – is one such example. Indeed, a deficiency of myostatin in humans (and animals) can lead to dramatic increases in muscle mass.
There are a range of methods to limit the abundance of a given protein in a cell, from removing the entire gene to turning off its expression, also known as “gene silencing”. As an example of the effect of this approach, researchers introduced molecules into mice which cut and degraded the RNA transcripts which otherwise would have resulted in the production of the myostatin protein. As a result, the researchers observed a significant reduction in the presence of myostatin for several weeks, with a 20% increase in muscle cross sectional area.
The images set out below, from another mouse study using different techniques, illustrate the extreme effect of completely “knocking out” the myostatin gene – the myostatin “knock out” mouse appears on the right (this mouse also has an overexpressed follistatin gene):
Comparison of a (a) wild type mouse (left), against (b) a mouse with a “knocked out” myostatin gene and overexpressed follistatin gene (right)

What are the deterrents to gene doping?
Those athletes who succumb to the pressure to dope risk both (a) being caught and banned (with catastrophic effects on their career), and (b) seriously and permanently damaging their health – these factors are explored further below.
Detecting gene doping and the associated sanction
Responding to the growing threat of gene doping, in 2003, WADA included in its Prohibited List the use of gene therapy techniques and set up its expert group on gene doping, which “gives direction […] in relation to the threat of gene doping by developing strategies to prevent and detect non-therapeutic manipulation of gene/protein in sport”.
The 2020 Prohibited List includes a broad prohibition of the different potential forms of gene doping with the following wording (where the term “nucleic acids” includes DNA and RNA):
The following, with the potential to enhance sport performance, are prohibited:
1. The use of nucleic acids or nucleic acid analogues that may alter genome sequences and/or alter gene expression by any mechanism. This includes but is not limited to gene editing, gene silencing and gene transfer technologies.
2. The use of normal or genetically modified cells.
If gene doping is detected, the default ban is four years. However, the detection of gene doping is not so simple, and this would likely be part of its appeal to would-be dopers. An example of a challenge that anti-doping organisations face in this respect is that proteins resulting from gene doping can be very similar, if not identical, to those produced naturally. Even if proteins resulting from gene doping are not identical to their natural counterparts, they may not make their way into the urine and blood, which are the sample types traditionally used in doping control testing. In this respect, Professor Sweeney, who sits in WADA’s gene doping expert group, has said:
I personally think I can prove to [World Anti-Doping Agency], if they really want the challenge, that I can dope dogs and they will never figure out which dogs were doped unless they take tissue biopsies…
In short, WADA will face many such challenges if gene doping becomes a reality.
The health risk deterrent
The majority of gene therapy techniques being developed are for the treatment of potentially fatal diseases where the tolerance of potential side effects and risks may be high. However, history tells us that potential side effects and risks may not necessarily put off those determined to use prohibited methods in the pursuit of sporting glory. The potential health risks of gene doping will depend on the technique used and the gene(s) targeted. However some examples of those risks are described below:
- The use of a viral vector to deliver genetic material can trigger a violent immune response. Those involved in gene therapy have been proceeding with extreme caution since a 1999 gene therapy trial in which one volunteer suffered an immune response so severe that it led to multiple organ failure and ultimately death. In one EPO study, monkeys injected with a new EPO gene via a viral vector developed severe anaemia.
- Gene therapy techniques can cause “off-target” effects where the activity of genes, which were not the intended targets, are altered. This could, for example, lead to the proliferation of cancer-causing cells or otherwise disrupt essential cellular functions.
- The over or under expression of certain genes which encode proteins associated with potential performance enhancement in sport can have catastrophic effects. By way of examples: (a) the overexpression of EPO can dramatically increase the viscosity of the blood and obstruct regular blood flow, and (b) the under expression of myostatin can lead to a rapid increase in muscle mass without cardiac adaptation, therefore increasing the risk of a heart attack.
These are just some of the health risks associated with techniques developed by reputable laboratories for legitimate purposes. If gene doping does become a reality, the gene doping products produced and supplied by unregulated and unaccountable underground laboratories are likely to present even more risks.
The future
Two decades after the publication of Sweeney’s IGF-1 gene research, there is some way to go before gene therapy becomes a routine treatment. That said, progress is being made and the relatively short list of gene therapies approved by the FDA is getting longer. However, the darker side to the promise of gene therapy technology is the potential for its abuse in sport, despite the potential risks.
This article was written by Richard Martin (Partner), who has a degree in Genetics from Nottingham University.
Footnote
1. Barton-Davis ER, et al. Viral mediated expression of insulin-like growth factor I blocks the aging-related loss of skeletal muscle function. Proc Natl Acad Sci U S A (1998) 95(26): 15603-7.
2. See: (a) http://www.thetimes.co.uk/tto/sport/article2246003.ece; and (b) http://www.dw.com/en/german-coach-suspected-of-genetic-doping/a-1890782
3. Wenner. How to Be Popular during the Olympics: Be H. Lee Sweeney, Gene Doping Expert. Scientific American 15 August 2008 http://www.scientificamerican.com/article/olympics-gene-doping-expert/
4. As described below, Class M3 of WADA’s Prohibited List identifies what WADA considers to amount to “gene and cell doping”.
5. Zhou S, et al. Adeno-associated virus-mediated delivery of erythropoietin leads to sustained elevation of hematocrit in nonhuman primates. Gene Therapy (1998) 5:665–670.
6. Zou Y, et al. Incorporation of a skeletal muscle-specific enhancer in the regulatory region of Igf1 upregulates IGF1 expression and induces skeletal muscle hypertrophy. Scientific reports (2018) 8:2781.
7. https://www.theguardian.com/science/2018/nov/26/worlds-first-gene-edited-babies-created-in-china-claims-scientist
8. Khan T, et al. Silencing myostatin using cholesterol-conjugated siRNAs induces muscle growth. Mol Ther Nucleic Acids (2016) 5(8):e342.
9. Lee SJ, Quadrupling muscle mass in mice by targeting TGF-ß signaling pathways. PLoS ONE (2007) 2(8): e789
10. https://www.wada-ama.org/en/gene-doping-expert-group
11. Wenner. How to Be Popular during the Olympics: Be H. Lee Sweeney, Gene Doping Expert. Scientific American 15 August 2008 http://www.scientificamerican.com/article/olympics-gene-doping-expert/
12. Lehrman, S. (1999) Virus treatment questioned after gene therapy death. Nature News. 401:517-518.
13. Gao et al. Erythropoietin gene therapy leads to autoimmune anaemia in macaques. Blood. 2004 May 1;103(9): http://www.bloodjournal.org/content/103/9/3300?sso-checked=true


